Lead (Ii) Nitrate + Hydrochloric Acid .hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. The balanced equation will be.
from mammothmemory.net
2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s). Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be.
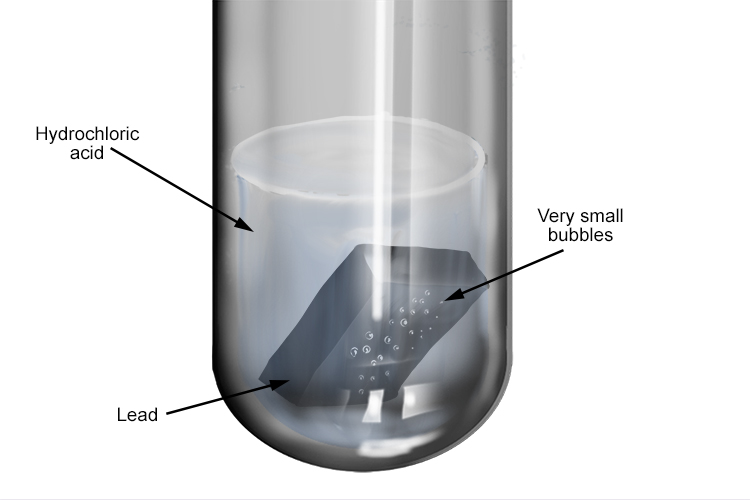
Leads reaction with hydrochloric acid is very slow indeed
Lead (Ii) Nitrate + Hydrochloric Acid 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s).hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. this video is the practical demonstration of the reaction of.lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate.
From www.bartleby.com
Answered (d) ammonium carbonate and hydrochloric… bartleby Lead (Ii) Nitrate + Hydrochloric Acid Enter an equation of an ionic chemical equation and press the balance button.hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. this video is the practical demonstration of the reaction of. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. balance the equation with one mole of lead nitrate, or pb (no3)2,. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.slideserve.com
PPT Chapter 8 Chemical Equations PowerPoint Presentation, free Lead (Ii) Nitrate + Hydrochloric Acid this video is the practical demonstration of the reaction of. 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s).lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. The balanced equation will be. Hcl + pb(no3)2 =. Lead (Ii) Nitrate + Hydrochloric Acid.
From chemistry.stackexchange.com
chemistry Explanation for why nickel turns green in Lead (Ii) Nitrate + Hydrochloric Acid 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s).hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid.lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. this video is the practical demonstration of. Lead (Ii) Nitrate + Hydrochloric Acid.
From tiannagroharvey.blogspot.com
Aqueous Sodium Chloride Reacts With Aqueous Lead Ii Nitrate Lead (Ii) Nitrate + Hydrochloric Acid this video is the practical demonstration of the reaction of. 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s).reaction between hydrochloric acid and lead nitrate:lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Web. Lead (Ii) Nitrate + Hydrochloric Acid.
From brainly.in
the reaction between red lead and hydrochloric acid is given as red Lead (Ii) Nitrate + Hydrochloric Acid Pb (no3)2(aq) + 2hcl(aq) → pbcl2(s) + 2hno3(aq) net. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement.reaction between hydrochloric acid and lead nitrate:lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. Enter an equation of an ionic chemical equation and press the balance. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.youtube.com
Silver Nitrate and Hydrochloric Acid YouTube Lead (Ii) Nitrate + Hydrochloric Acid Pb (no3)2(aq) + 2hcl(aq) → pbcl2(s) + 2hno3(aq) net. Enter an equation of an ionic chemical equation and press the balance button. this video is the practical demonstration of the reaction of. 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s). The balanced equation will be. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.carolina.com
Lead (II) Nitrate Solution, 0.5 M Aqueous, Laboratory Grade, 500 mL Lead (Ii) Nitrate + Hydrochloric Acidlead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. The balanced equation will be. 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s).hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. balance the. Lead (Ii) Nitrate + Hydrochloric Acid.
From favpng.com
Lead(II) Nitrate Crystal Structure, PNG, 1100x1099px, Leadii Nitrate Lead (Ii) Nitrate + Hydrochloric Acidlead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. The balanced equation will be. Enter an equation of an ionic chemical equation and press the balance button. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement.reaction between hydrochloric acid and lead nitrate: Lead (Ii) Nitrate + Hydrochloric Acid.
From mammothmemory.net
Leads reaction with hydrochloric acid is very slow indeed Lead (Ii) Nitrate + Hydrochloric Acid Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. balance the equation with one mole of lead nitrate, or pb (no3)2, with two moles of hydrochloric acid, or hcl, to. this video is the practical demonstration of the reaction of. The balanced equation will be. Pb (no3)2(aq) + 2hcl(aq) → pbcl2(s) + 2hno3(aq) net. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Lead (Ii) Nitrate + Hydrochloric Acid this video is the practical demonstration of the reaction of.reaction between hydrochloric acid and lead nitrate: Pb (no3)2(aq) + 2hcl(aq) → pbcl2(s) + 2hno3(aq) net.hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. Enter an equation of an ionic chemical equation and press the balance button. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.chegg.com
Solved 3, lead(II) nitrate + potassium sulfate ? (NOs )2 4 Lead (Ii) Nitrate + Hydrochloric Acid 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s). The balanced equation will be. Hcl + pb(no3)2 = pbcl2 + hno3 is a double displacement. this video is the practical demonstration of the reaction of. Enter an equation of an ionic chemical equation and press the balance button. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for HCl + MgCO3 = MgCl2 + CO2 + H2O Lead (Ii) Nitrate + Hydrochloric Acid Pb (no3)2(aq) + 2hcl(aq) → pbcl2(s) + 2hno3(aq) net. 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s).lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate.reaction between hydrochloric acid and lead nitrate: The balanced equation. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.youtube.com
Lead Nitrate + Hydrochloric Acid YouTube Lead (Ii) Nitrate + Hydrochloric Acidlead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate.hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. this video is the practical demonstration of the reaction of. Enter an equation of an ionic chemical equation and press the balance button. balance the equation. Lead (Ii) Nitrate + Hydrochloric Acid.
From fphoto.photoshelter.com
science chemical reaction equilibrium hydrochloric acid Fundamental Lead (Ii) Nitrate + Hydrochloric Acid this video is the practical demonstration of the reaction of.reaction between hydrochloric acid and lead nitrate: 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s). The balanced equation will be. Pb (no3)2(aq) + 2hcl(aq) → pbcl2(s) + 2hno3(aq) net. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.toppr.com
State your observations when(i) Dilute hydrochloric acid is added to Lead (Ii) Nitrate + Hydrochloric Acidhydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid. this video is the practical demonstration of the reaction of. The balanced equation will be. Enter an equation of an ionic chemical equation and press the balance button. balance the equation with one mole of lead nitrate, or pb (no3)2, with two moles of hydrochloric acid,. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead (Ii) Nitrate + Hydrochloric Acid balance the equation with one mole of lead nitrate, or pb (no3)2, with two moles of hydrochloric acid, or hcl, to.lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate. this video is the practical demonstration of the reaction of. The balanced equation will be.reaction. Lead (Ii) Nitrate + Hydrochloric Acid.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Lead Nitrate And Hydrogen Chloride Lead (Ii) Nitrate + Hydrochloric Acid Pb (no3)2(aq) + 2hcl(aq) → pbcl2(s) + 2hno3(aq) net. this video is the practical demonstration of the reaction of. balance the equation with one mole of lead nitrate, or pb (no3)2, with two moles of hydrochloric acid, or hcl, to. 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟. Lead (Ii) Nitrate + Hydrochloric Acid.
From www.chegg.com
Solved How many grams of lead chloride are produced from Lead (Ii) Nitrate + Hydrochloric Acid 2 hcl (aq.) ⏟ hydrochloric acid + pb (no 3) 2 (aq.) ⏟ lead nitrate → pbcl ⏟ 2 (s).hydrogen chloride + lead(ii) nitrate = lead chloride + nitric acid.reaction between hydrochloric acid and lead nitrate: The balanced equation will be. this video is the practical demonstration of the reaction of. Lead (Ii) Nitrate + Hydrochloric Acid.